Tech & Tools
Technical information
- HOME
- Tech & Tools
- Calculation of chemical / carbon dioxide injection amount
Calculation of chemical / carbon dioxide injection amount
Calculation of chemical injection amount
The theoretical required amount of chemical injection required for pH neutralization is calculated by the following formula. However, in reality, it is not neutralized by the theoretical dose. Use the calculation result multiplied by the margin of 3 to 5 as a guideline for the amount of drug to be injected.
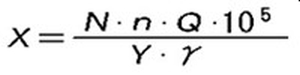
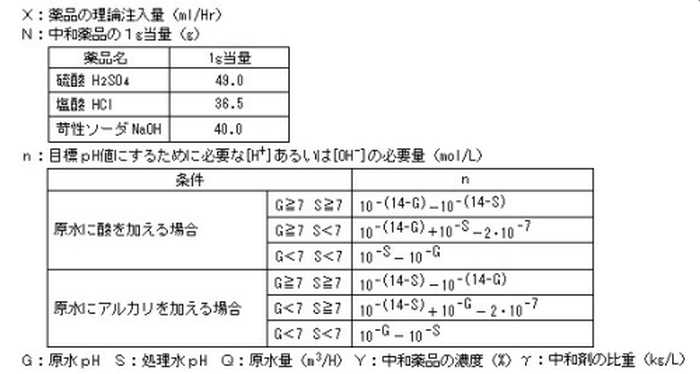
The actual amount of chemicals required for neutralization must be confirmed by a neutralization titration test using raw water.
Please contact us if you would like a neutralization titration.
Calculation of carbon dioxide injection amount
Assuming that the alkali contained in the raw water is caustic soda [NaOH]
CO2 [carbon dioxide] + H2O [water] = H2CO3 [carbonic acid]
2NaOH [caustic soda] + H2CO3 [carbonic acid] = Na2CO3 [sodium carbonate] + 2H2O [water]
Na2CO3 [sodium carbonate] + H2CO3 [carbonic acid] = 200973 [sodium bicarbonate]
To summarize the above
2CO2 + 2NaOH = 200973
Therefore, 1 mol of carbon dioxide is required to neutralize 1 mol of caustic soda.
For example, the amount of OH- contained in raw water with a pH of 10.8 at 3 m3 / Hr is 10-3.2 [mol / L].
The amount of OH- contained in 3m3 / Hr of raw water is 3000L / Hr x 10-3.2 = 1.9 mol / Hr.
Carbon dioxide 44 g / mol x 1.9 mol / Hr x margin 5 = 418 g / Hr
The gas injection amount is [418g / Hr x 1000mL / 44g] x 22.4 = 212800ml / Hr ≒ 213 L / Hr
Therefore, when carbon dioxide is added to raw water [alkali], the gas injection amount is 213 L / Hr.
* If the alkali contained in the raw water is a polyvalent alkali such as calcium hydroxide Ca [OH] 2, theoretically, the amount of gas required is several times the valence.
Head Office
1-12-11 Tagawakita, Yodogawa-ku,Osaka
532-0021
Overseas Business Department
TEL +81-6-6301-6460
FAX +81-6-6308-3022




