Tech & Tools
Technical information
- HOME
- Tech & Tools
- pH measurement
pH measurement
There are three main methods for measuring pH.
<Indicator method>
In the indicator method, a buffer solution is used to compare the standard color at each pH value with the color of the measurement solution + buffer solution. Another method is to soak the buffer solution in paper [litmus test paper], immerse the paper in the measurement solution, _and check the color development. Both methods can only be read roughly, so the measurement is for reference only.
* Buffer solution: A solution that keeps the hydrogen ion concentration almost constant even when acids _and bases are added from the outside.
<Metal electrode method>
The metal electrode method includes the hydrogen electrode method, the quinhydrone electrode method, _and the antimony electrode method. The hydrogen electrode method is the most reliable measurement method that serves as a standard for various pH measurements. An electrode using platinum or hydrogen gas is immersed in a measurement solution for measurement. However, it is difficult to measure because it is inconvenient to handle hydrogen gas _and it is easily affected by substances with strong oxidizing _and reducing properties, so it is a strict measurement method for regular use. The quinhydrone electrode method _and antimony electrode method are rarely used at present due to factors such as the problem of the measurable pH range _and the change in the measured value depending on the electrode condition.
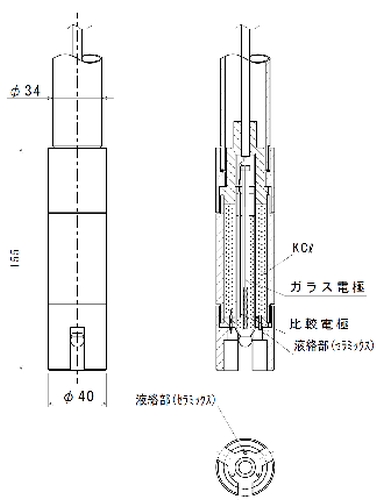
<Glass electrode method>
The glass electrode method is the most widely used measurement method in current pH measurement because it has a fast measurement reaction _and good reproducibility. In addition, it is not affected by oxidizing / reducing agents _and has the advantage of not being affected by any measurement target, _and our pH electrode also uses this method.
This is a measurement method that uses two electrodes, a glass electrode _and a comparison electrode, to detect the voltage [potential difference] generated between these two electrodes _and convert it into a pH value. When there are solutions with different pH inside _and outside the thin film glass, an electromotive force proportional to the difference in pH is generated in the thin film part, _and the pH value is calculated by measuring it.
Head Office
1-12-11 Tagawakita, Yodogawa-ku,Osaka
532-0021
Overseas Business Department
TEL +81-6-6301-6460
FAX +81-6-6308-3022




