Tech & Tools
Technical information
- HOME
- Tech & Tools
- Manganese removal mechanism
Manganese removal mechanism
The manganese-removing filter material (manganese sand, ferolite GC, ferolite MC) uses filtered sand (manganese sand), ceramics G (ferrorite GC), and ceramics S (ferrorite MC) as carriers, and potassium permanganate. , Manganese sulfate, manganese chloride are alternately reacted to produce hydrated manganese dioxide (MnO2, nH2O), which has a special structure.
● Manganese removal reaction
The general reaction formula for catalytically removing manganese in water is:
Mn2+ + NaClO + 2H2O → 2MnO2・H2O + NaCl + 2H+
The reaction from the viewpoint of the manganese-free filter medium is explained in the following figure. As shown in equation (1), if the raw water containing Mn2+ touches the surface of MnO2/H2O, Mn2+ ions exchange with H+ ions.
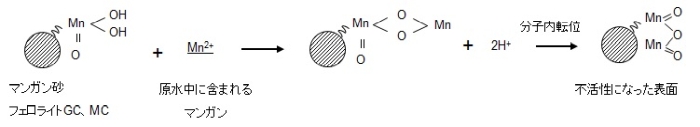
Since this ion exchange has a finite number of exchange groups, it should not be able to catch Mn2+ in due course, but in reality, the manganese removal capacity is permanently maintained and it is continuous. The manganese removal reaction proceeds. This is because the contact surface becomes the original MnO2・H2O due to the oxidative regeneration reaction that occurs in the presence of sodium hypochlorite as shown in equation (2).This is called an autocatalytic reaction.
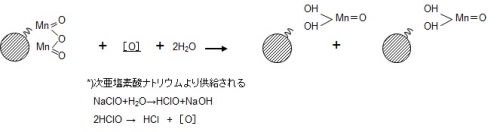
Since manganese is continuously oxidized by an autocatalytic reaction, manganese in raw water can be continuously removed only with an oxidizing agent such as sodium hypochlorite. However, since raw water contains reducing substances such as turbid substances and organic substances, it is necessary to replace the filter medium every few years in order to obtain stable treated water.
[Reference] by Yu Takai and Hiroshi Nakanishi, “Iron removal and manganese removal treatment for water”
Head Office
1-12-11 Tagawakita, Yodogawa-ku,Osaka
532-0021
Overseas Business Department
TEL +81-6-6301-6460
FAX +81-6-6308-3022






